Would you like to take part in a Clinical Trial for a new investigational treatment for Atopic Dermatitis or Eczema at no cost to you?
No health insurance is required to participate. You will receive all study related care from a specialist physician at no cost. The study will include visits to a clinic in your location.
You may be eligible to participate in the study if:
- You are over the age of 18
- You have Eczema or Atopic Dermatitis
Sign Up
What happens if I Sign Up?
We will match you to a research study location in your area that needs volunteers with Eczema (Atopic Dermatitis) or notify you when one becomes available. The study team will then contact you and you may have the opportunity to participate if qualified.
If you think you might like to participate in the Eczema (Atopic Dermatitis) Studies or would like more information, please enter your information below so we can confirm if you may qualify and can contact you about the study. Keep in mind that participation is entirely voluntary. If you do decide to take part in the study, you are free to change your mind about participating at any time.
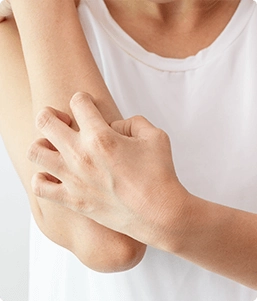
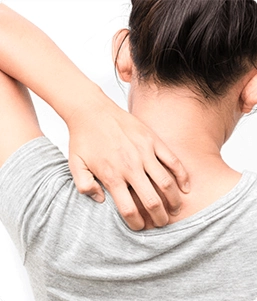
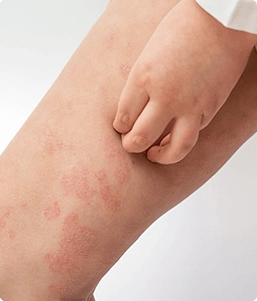
About Eczema
Eczema (Atopic Dermatitis) is a chronic condition that makes the skin red and itchy. Atopic Dermatitis (or Eczema) tends to be more common in children, but people of any age are susceptible to this condition.1 There is no cure, but there are numerous treatment options available such as topical creams or ointments. There are other self-care tips that one can use in order to combat the symptoms, such as keeping the skin moisturized and avoiding harsh soaps and creams.
Symptoms to look out for are:
- Dry skin
- Thick, cracked or scaly skin
- Red or brown/grey patches on the skin
- Itching (usually more severe at night)
- Bumpy "chicken skin"
Frequently Asked Questions
What is a clinical trial or research study?
A clinical trial, also referred to as a research study, is a scientific study that evaluates the safety and efficacy of an investigational medication. A research study may show that the investigational medication is better than, as good as, or worse than the standard treatment or inactive placebo. Qualified doctors, nurses and other medical professionals will conduct the study.
It is only through the completion of research studies that investigational medication can be evaluated, and if proven safe and effective, approved for general use by appropriate regulatory or health authorities, such as the U.S. Food and Drug Administration (FDA). Prescription medications in use today were first proven safe and effective in research studies.
What will the Eczema Studies involve?
Participation in the Eczema Studies will involve visits to a study clinic near your home.
Those who qualify will receive either the investigational drug or a placebo, as well as all call and study-related medical exams and laboratory tests, all at no cost. Compensation for travel may also be available.
In the Eczema Studies, participants will be randomly assigned to a study group. You will receive either the investigational drug or a placebo, depending on the assigned study group. Neither you nor the study team will know which study group you are in, but in the case of an emergency, the study doctor can quickly find out.
Will I be reimbursed for study-related travel?
Volunteers who qualify to take part in the study may receive compensation for study-related travel. Please discuss this with the study team when they contact you.
Is there a cost to participate?
There is no cost to participate in the Eczema Studies. If you qualify, all study-related visits, tests, care, and study drug will be provided at no cost.
If you decide to take part, you will receive study-related care throughout the study from a team of experienced doctors and nurses.
What else do I need to consider?
The research team will be able to explain more about what the Eczema Studies will involve, and it is up to you to decide if you want to take part. Participation is voluntary, whether or not you decide to participate will not affect your current or future relationships with your doctors. If you decide to participate, you are free to withdraw at any time without affecting those relationships or the care you receive.
How far is the study clinic?
We match you to a study clinic within a close travel distance from your home. If we are not running the study in your area currently, with your permission, we will keep you in our database and reach out once a study clinic in your area becomes available. If, at any time, you decide you no longer want to participate in the study, you can opt out and we will delete your information.
What happens to personal information?
When you sign up for a research study, your personal information is protected as required by law. The research team stores personal and private information with codes (instead of names or other identifying information), in order to not identify the participant or volunteer. The informed consent form that will be provided to you by the research team will have more information about privacy protection.
